- Home
- Latest News
Latest News
Latest news and updates on different dimensions of Corporate Social Responsibility (CSR) in India and abroad.
Know more - Interviews
- Events - CASCA 2026
Events - CASCA 2026
Know more- Climate Action and Sustainability Conference & Awards 2026
- Climate Action & Sustainability Conference & Awards 2025
- Social Impact Conference & Awards 2025
- ESEC 25 - Education, Skilling & Employability Conference 2025
- Social Impact Conference and Awards 2024
- Social Impact Conference and Awards 2023
- Social Impact Conference and Awards 2022
- COVID Response Social Impact Awards 2021
- Insights
Insights
Find in-depth insights on CSR knowledge, latest CSR policies, expert opinion and special reports
Know more - Sustainability
- YourVoice
- Organisations
- Reports
- Jobs
- Fellowships
- Partner With Us
- Videos
Never miss the latest ESG news, interviews & insights. Subscribe for our weekly newsletter!







.jpg)



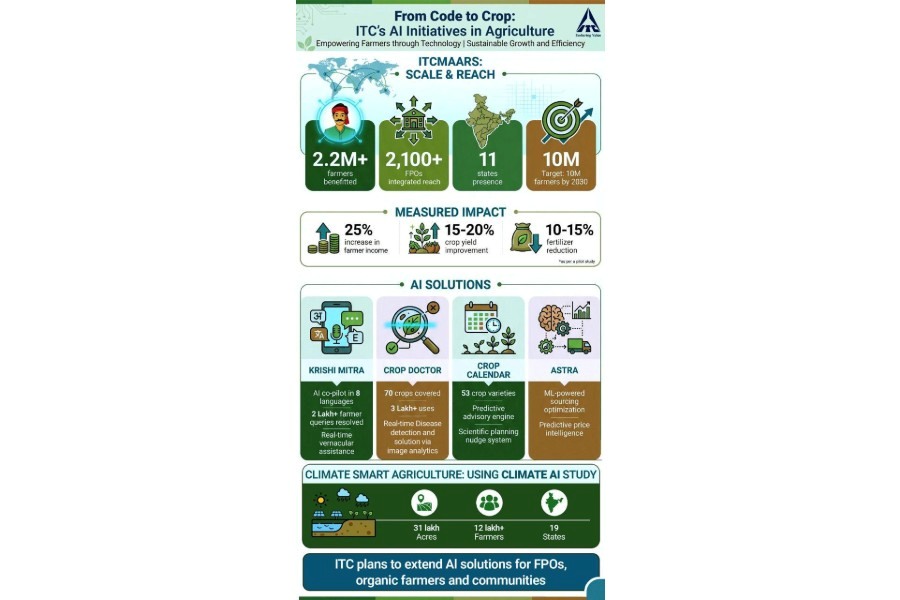








.jpg)



